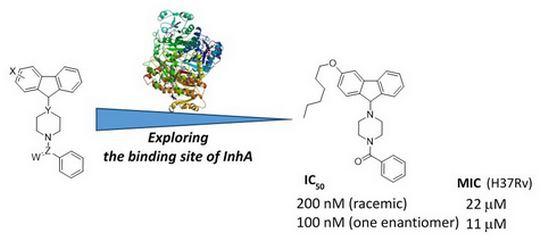
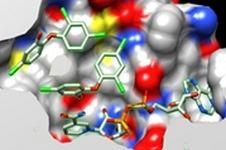
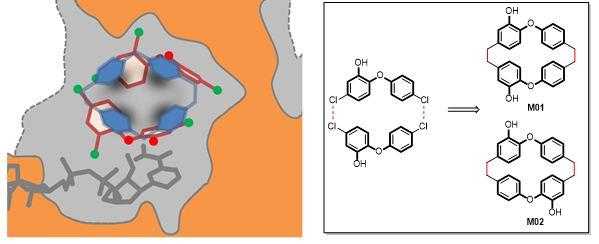
Collaborations
- Dr. L. Mourey, Pr. L. Maveyraud (IPBS, Toulouse)
- Pr. J. Korduláková (Bratislava, Slovaquie)
- Pr. M. R. Pasca, Pr. G. Degiacomi (Pavie, Italie)
- Pr. Ch. Laurent (CIRIMAT, Toulouse)
Funding
- UFP-MiP Région Occitanie
- SATT Toulouse Tech Transfer
- ANR TagInhA (2023-2026)